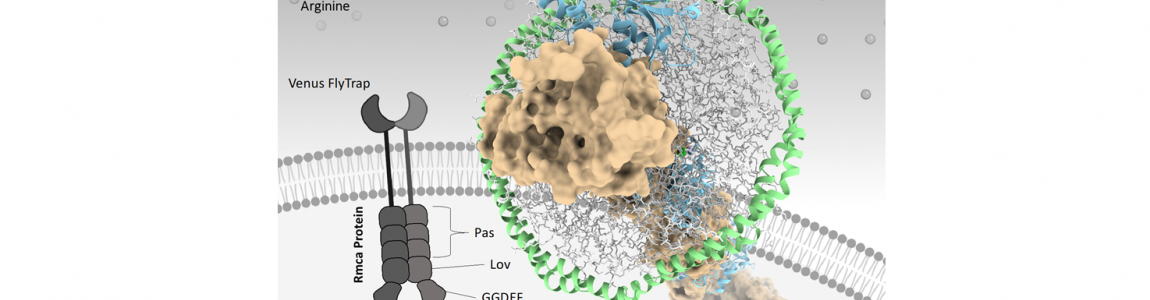
Nutrient sensing and redox transducers in bacterial biofilm.
Bacteria can behave as multicellular community named biofilm, which confer them protection against host defense and hostile environments. The switch between planktonic to biofilm lifestyle is controlled by the variation of the intracellular levels of the second messenger bis-(3′,5′)-cyclic-dimeric-guanosine (c-di-GMP). C-di-GMP levels control the metabolic re-programming required for biofilm formation and dispersion (the latter upon decrease of c-di-GMP) in response to environmental cues, including electron acceptors and nutrients. Among nutrients, arginine represents one key metabolite in biofilm formation being at the crossroad of many metabolic processes and acting as a substrate for nitric oxide production by the host immune system.
We are currently working on an arginine dependent transducer able to integrate arginine perceiving and electrons availability to finely tune c-di-GMP; this redox-mediated control joins the environmental sensing (i.e. nutrient) with the respiratory capability, to finally re-shape biofilm morphology according to the nutritional and energetic needs. Other nutrient-dependent transducers, coping with c-di-GMP, are currently being characterized. The role of nutrient supplementation on the energy metabolism has been analyzed in a real-time fashion by means of Seahorse measurements.
Protein engineering, membrane protein purification, nanodisc technology, molecular biophysics and enzyme kinetics are the main approaches used to characterize this kind of transducers.
See the other infos for the specific know-how and the technical approach.

